Unexpected size dependence of the sticking coefficient
Particle deposition to water-solid interfaces has been studied by numerous authors, us included [1-5]. When the particle coverage is low, the deposition is a first order kinetic process in the particle concentration. The corresponding deposition rate coefficient k can be measured for example with reflectivity in a straightforward fashion. In case of favorable deposition, attractive forces between the particles and the substrate induce that the approaching particle sticks immediately to the surface when it is in its proximity. In this situation, the deposition rate coefficient kcal has also been calculated theoretically in the impinging jet geometry by assuming perfect sink conditions at the surface [6]. A common way to analyze experimental data is to report the sticking coefficient
β = k/kcal . In many situations, this coefficient turns out to be close to unity, typically in the range 0.3-0.6. This minor discrepancy can be explained by hydrodynamic interactions between the depositing particle and the surface.
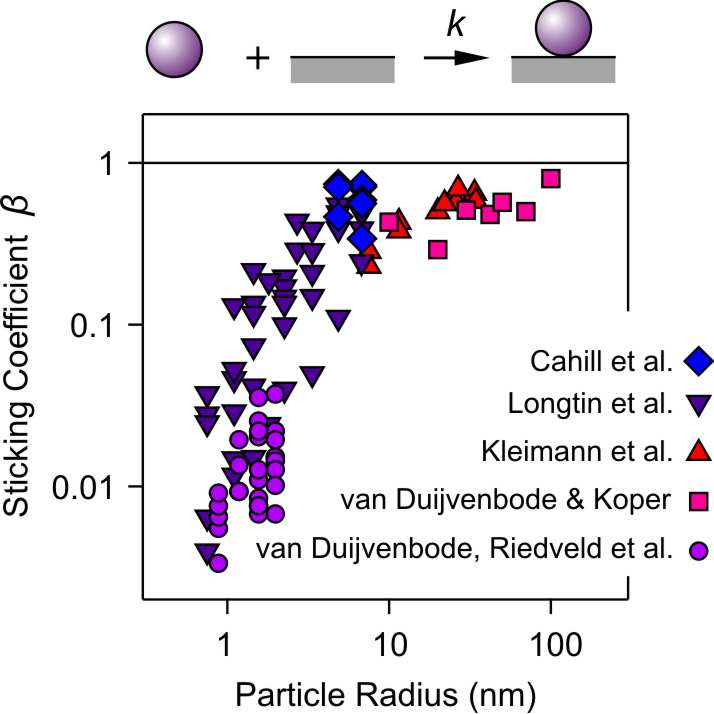 When one investigates the deposition of nanosized particles, the sticking coefficient may turn out to be substantially smaller than unity. This effect was reported for poly(propylene imine) dendrimers [4] and subsequently for larger latex particles [3]. We have also observed similar trends with nanosized latex particles [2]. We have also investigated this effect with poly(amido amine) (PAMAM) dendrimers. Larger dendrimers showed unsuspicious behavior [4], but when the studies were extended to smaller dendrimers [5], a strong dependence on the size of these particles was observed. The data known to us are summarized in the figure below. The sticking coefficient decreases with decreasing particle size, especially below 10 nm. For latex particles it was suggested that the dependence on their size originates from surface charge heterogeneities [2], but this explanation will apply unlikely to the rather homogeneous dendrimers. So far, we were unable to find a plausible explanation for this behavior.
When one investigates the deposition of nanosized particles, the sticking coefficient may turn out to be substantially smaller than unity. This effect was reported for poly(propylene imine) dendrimers [4] and subsequently for larger latex particles [3]. We have also observed similar trends with nanosized latex particles [2]. We have also investigated this effect with poly(amido amine) (PAMAM) dendrimers. Larger dendrimers showed unsuspicious behavior [4], but when the studies were extended to smaller dendrimers [5], a strong dependence on the size of these particles was observed. The data known to us are summarized in the figure below. The sticking coefficient decreases with decreasing particle size, especially below 10 nm. For latex particles it was suggested that the dependence on their size originates from surface charge heterogeneities [2], but this explanation will apply unlikely to the rather homogeneous dendrimers. So far, we were unable to find a plausible explanation for this behavior.
Michal Borkovec, April 29, 2014.
References
[1] R. C. van Duijvenbode, I. B. Rietveld, G. J. M. Koper (2000) Light reflectivity study on the adsorption kinetics of poly(propylene imine) dendrimers on glass, Langmuir 16, 7720-7725, 10.1021/la0004552.
[2] R. C. van Duijvenbode, G. J. M. Koper (2001) Fast adsorption on nonideal surfaces, J. Phys. Chem. B 105, 11729-11736, 10.1006/jcis.2001.7569.
[3] Kleimann J., Lecoultre G., Papastavrou G., Jeanneret S., Galletto P., Koper G. J. M., Borkovec M. (2006) Deposition of nanosized latex particles onto silica and cellulose surfaces studied by optical reflectometry, J. Colloid Interf. Sci. 303, 460-471, 10.1016/j.jcis.2006.08.006.
[4] Cahill B. P., Papastavrou G., Koper G. J. M., Borkovec M. (2008) Adsorption of poly(amido amine) (PAMAM) dendrimers on silica: Importance of electrostatic three-body attraction, Langmuir, 24, 465-473, 10.1021/la7021352.
[5] Longtin R. and Maroni P. (unpublished)
[6] T. Dabros, T. G. M. van de Ven (1983) A direct method for studying particle deposition onto solid surfaces, Colloid Polym. Sci. 261, 694-707, 10.1007/BF01415042.
