Discrepancies in electrical surface potentials
A charged water-solid interface in contact with an electrolyte solution is referred to as the electrical double layer. Within the double layer, one layer refers to the charges at the solid surface, and the other to the diffuse layer in solution. Since there is a finite gap between the charge of the solid and the plane of origin of the diffuse layer, the diffuse layer potential is normally smaller than the potential of the solid surface. This difference can be enhanced by the presence of tightly bound ions to the surface.
A common way to estimate the diffuse layer potential is by electrophoresis. In the case of colloidal particles, one can measure the electrophoretic mobility (EM) in an electric field, and by invoking the standard electrokinetic model one can evaluate the surface potential, which is normally referred to as the ζ-potential. For weakly charged surfaces, the surface potential is proportional to the mobility.
For more highly charged surfaces, the relation is nonlinear, and can be calculated within the so-called standard electrokinetic model [1]. The plane of shear was suggested to be situated at some finite distance away from the surface, and this distance might be comparable to the size of few water molecules.
 One would thus expect that the magnitude of the ζ-potential should be in fact smaller as the one of the diffuse layer potential, or in the best case the same.
One would thus expect that the magnitude of the ζ-potential should be in fact smaller as the one of the diffuse layer potential, or in the best case the same.
Recently, an alternative way to measure the diffuse layer potential became available, namely by direct force measurements. One can measure the force between two charged colloidal particles with the atomic force microscope (AFM) [2,3]. From this force, one can extract the magnitude of the surface potential. For weakly charge surfaces, this force is proportional to the square of the surface potential.
With these two techniques at hand, one can measure the ζ-potential and the diffuse layer potential for the same particles independently. One would expect that these values should be the same, or eventually that the ζ-potential should be smaller in magnitude due to the finite distance of the plane of no shear. By combining these techniques, one should even be able to estimate the latter distance.
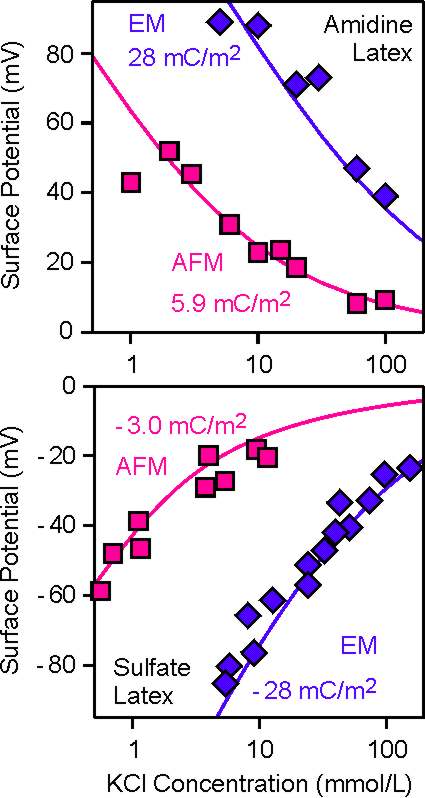
We compared the surface potentials determined by electrophoresis and by AFM force measurements, but the results caught us by surprise. The magnitude of the ζ-potential was actually larger than the diffuse layer potential measured by AFM. This effect was most pronounced for highly charged particle surfaces, while for weakly charged surfaces the agreement between these two techniques was very good [2,3]. The figures on the right show two examples of this behavior for bare sulfate and amidine latex particles.
Pavel Adam, Gregor Trefalt, and Michal Borkovec, April 29, 2014.
References
[1] Russel W. B., Saville D. A., Schowalter W. R.: Colloidal Dispersions. Cambridge: Cambridge University Press; 1989.
[2] Finessi M., Sinha P., Szilagyi I., Popa I., Maroni P., Borkovec M. (2011) Charge reversal of sulfate latex particles by adsorbed linear poly(ethylene imine) probed by multiparticle colloidal probe technique, J. Phys. Chem. B 115, 9098-9105, 10.1021/jp203514r.
[3] F. J. Montes Ruiz-Cabello, P. Maroni, M. Borkovec (2013) Direct measurements of forces between different charged colloidal particles and their prediction by the theory of Derjaguin, Landau, Verwey, and Overbeek (DLVO), J. Chem. Phys. 138, 234705, 10.1063/1.4810901.
