Strange stability plot
Suspensions of charged particles are stable in pure water, but they can be destabilized by addition of salt. The stability of such a suspension can be quantified by means of the stability ratio, which is inversely proportional to the aggregation rate. This quantity is normalized to unity at high salt concentration. At these conditions, the aggregation process is rapid, and only controlled by mutual diffusion of the particles. At lower salt levels, the aggregation is slower due to electrostatic repulsion between the particles, and the stability ratio becomes larger. The stability ratio can be measured by time-resolved dynamic light scattering. With increasing salt concentration, the stability ratio continuously decreases, and when it reaches unity, it remains constant. This behavior is in qualitative agreement with the classical theory of Derjaguin, Landau, Verwey, and Overbeek (DLVO). Numerous systems were shown to behave in this fashion [1,2]. One example is also given here.
When one adds a polyelectrolyte to an aqueous suspension of oppositely charged colloidal particles, the polyelectrolyte adsorbs strongly to the particle surface. At a moderate dose, the adsorbed polyelectrolytes neutralize the surface charge, but for a sufficiently large dose, the particle charge is reversed. At this point, the particle surface is decorated with a tightly bound polyelectrolyte layer, and this "dressed" particle behaves as any other charged particle. In many situations, the stability ratio versus the salt concentration of such polyelectrolyte-dressed particles looks pretty much the same as for bare particles [3].
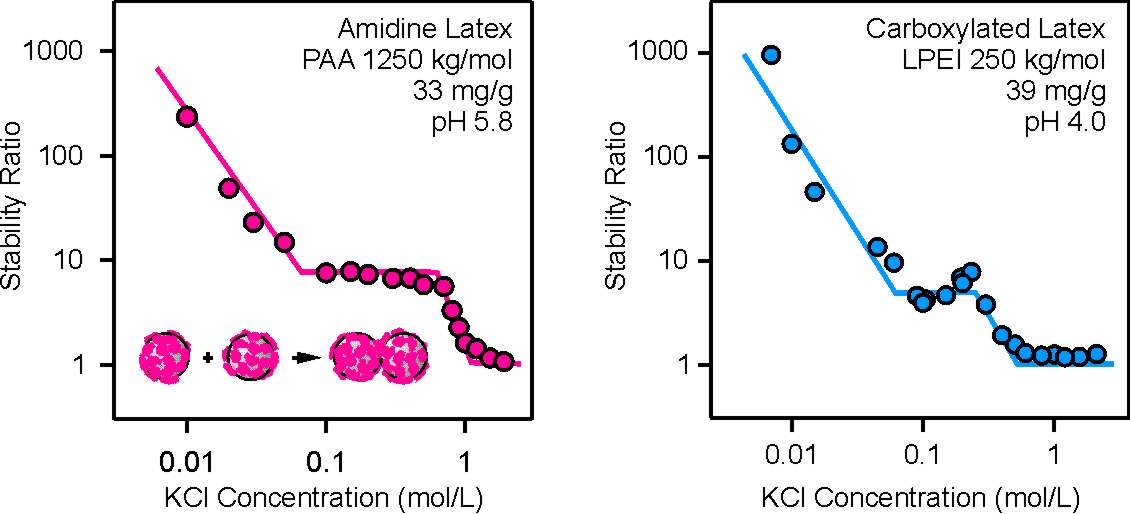
In some cases, however, such polyelectrolyte-dressed particles a somewhat different stability behavior is observed, see figures below. With increasing salt concentration, the stability ratio reaches an intermediate plateau. At this plateau, this ratio is independent of the salt level. Only at higher salt concentrations, the stability ratio decreases to unity. This behavior was observed in two different systems, namely for carboxylated latex particles of 310 nm in diameter and linear(polyethylene imine) (LPEI) and amidine latex particles of diameter 220 nm and poly(acrylic acid) (PAA). Under the experimental conditions, both polyelectrolytes were almost fully ionized. In both case, the dose was chosen such that a fully saturated layer forms. Additional experiments indicated that this plateau effect becomes more important for high molecular mass polyelectrolytes. The origin of this plateau is unclear to us.
Istvan Szilagyi and Michal Borkovec, April 29, 2014.
References
[1] Russel W. B., Saville D. A., Schowalter W. R.: Colloidal Dispersions. Cambridge: Cambridge University Press; 1989.
[2] Lin W., Kobayashi M., Skarba M., Mu C., Galletto P., Borkovec M. (2006) Heteroaggregation in binary mixtures of oppositely charged colloidal particles, Langmuir, 22, 1038-1047, 10.1021/la0522808.
[3] Hierrezuelo J., Sadeghpour A., Szilagyi I., Vaccaro A., Borkovec M. (2010) Electrostatic stabilization of charged colloidal particles with adsorbed polyelectrolytes of opposite charge, Langmuir 26, 15109-15111, 10.1021/la102912u.
