Science puzzles
Some scientists may pretend to be able to explain everything. Some of us do not quite share this opinion, not even within our own area of expertise. While we still are able to explain many observations on the spot, explaining others may make take a while. Often, we then succeed as well. However, there are some observations that we have failed to explain in a satisfactory fashion for years. These are our science puzzles, and you find some of them below.
| Fast aggregation too slow At high salt levels, colloidal particles aggregate rapidly, since their approach is mainly controlled by diffusion. By neglecting all interactions, Smoluchowski calculated the right order of magnitude of this rate. Including the know conservative and hydrodynamic forces in the calculation, the agreement experimental and calculated rates becomes better. However, an important discrepancy remains. More... |
|
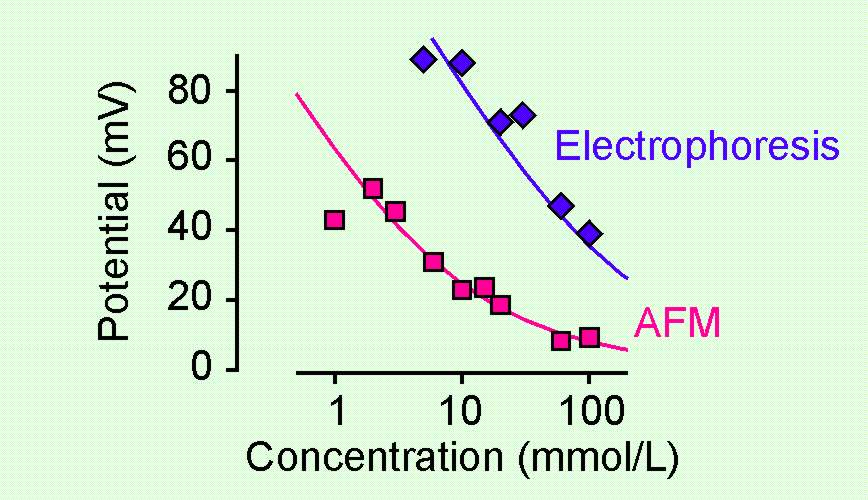
| Discrepancies in surface potentials The diffuse layer potential of a charged water-solid interface is normally measured by electrokinetic techniques. More recently, these potentials can also be accessed by direct force measurements. We have compared these two different techniques, to our great suprize these two technique may give quite different results. Especially, the potentials measured with electrokinetics may exceed in magnitude the ones obtained by AFM substantially. More... |
|
| Unexpected radius dependence When particles deposit under favorable conditions to water-solid interface, the deposition rate can be reliably predicted with the perfect sink model. For very small particles, however, the deposition rate is much smaller than expected. More... |
|
| Strange stability plot When charged colloidal particles are coated with oppositely charged polyelectrolytes, these coated particles behave in many respects as any other charged particle. However, some anomalies are observed in the stability of these particles. More... |

|
