Membrane-based water treatment to increase water supply
In separation applications using membranes, the goal is to allow one or more components of a mixture to permeate the membrane, while hindering permeation of other components. A normal filter meets this definition of a membrane, but, by convention, the term filter is usually limited to structures that separate components larger than roughly 1–10 μm. The term membrane is used for separation of smaller compounds.
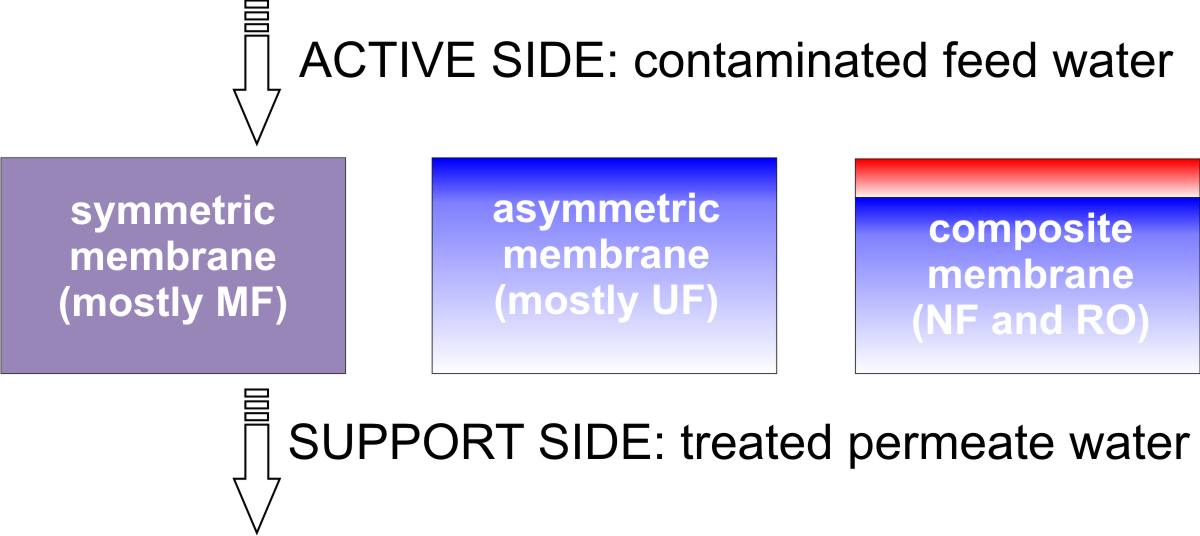
Membrane types. Most of the membranes used currently are anisotropic, see figure above [1,2]. These membranes comprise a dense layer formed over a thicker porous support layer. The separation properties and permeation rates of the membrane are determined exclusively by the surface layer, while the substructure functions as a mechanical support. When the material of the top layer and that of the porous sublayer are the same, the membrane is called an asymmetric membrane. Asymmetric polymeric membranes are usually fabricated by a phase separation process [3,4]. On the other hand, if the material of the top layer is different from the material of the porous sublayer, the membranes are called composite membranes, such as those currently used in nanofiltration (NF) and reverse osmosis (RO). The advantage of the composite membrane over the asymmetric membrane is that the material for the top skin layer and the porous sublayer can be chosen separately to optimize the overall performance [5].
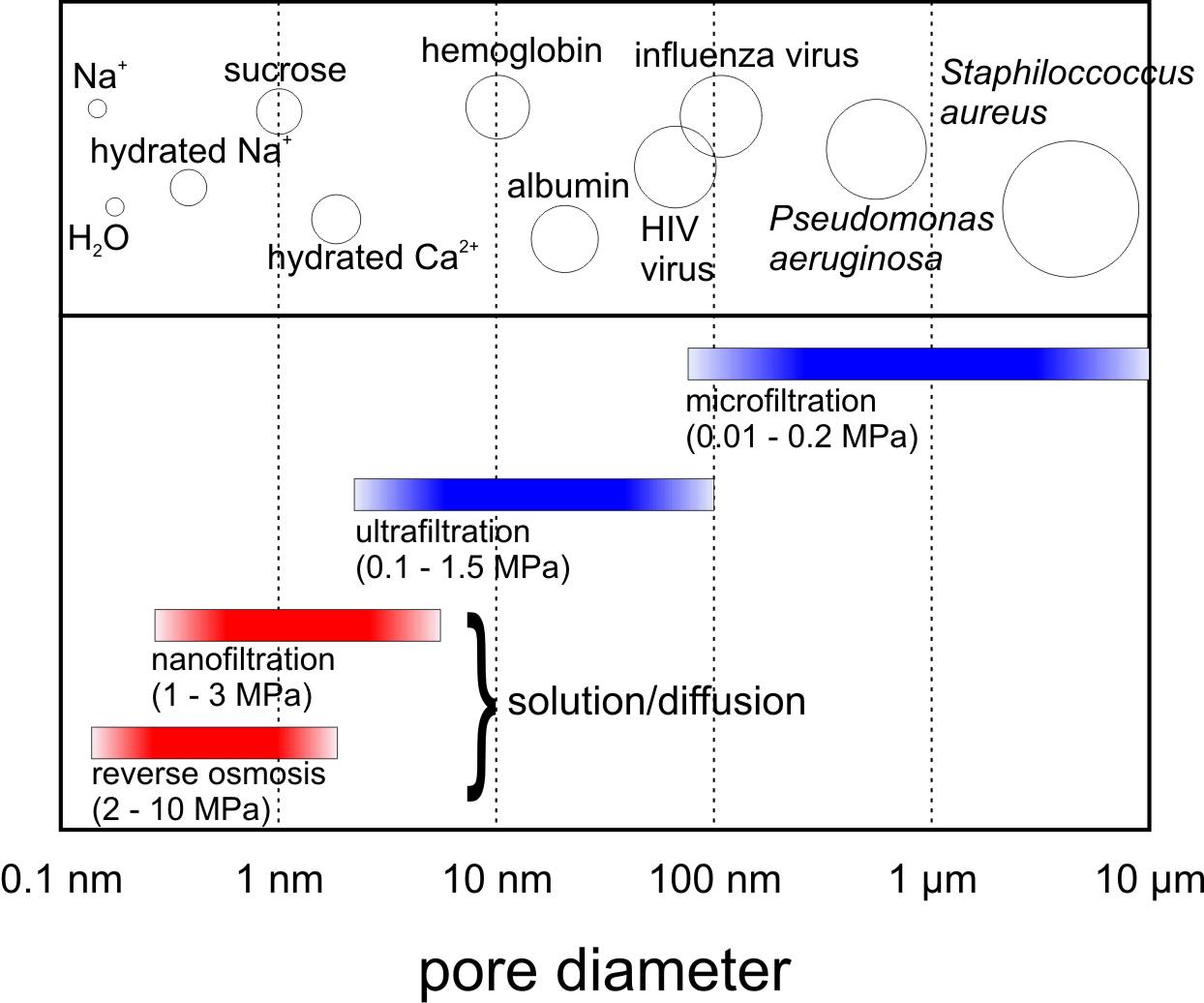 Membrane processes. Membrane processes are divided based on the membrane type, what it separates, the driving force used to create the chemical potential difference between the two sides of the membrane, and the transport mechanism [1,2]. Here, we will focus on separation driven by a hydraulic trans-membrane pressure. Microfiltration (MF) and ultrafiltration (UF) are similar in that the separation is mostly molecular sieving through the pores. These membranes are illustrated in the right figure. MF membranes are usually symmetric and can filter colloidal particles and bacteria from 0.1 to 10 μm in diameter. Therefore, they are used for clarification, sterilization, and to concentrate suspensions. In water treatment, MF is used to remove pathogens for disinfection and occasionally to remove turbidity and flocculants in wastewater treatment. UF membranes are generally asymmetric and they can be used to filter dissolved macromolecules, such as proteins, from solutions. While the primary separation mechanism in UF is size exclusion, other physicochemical interactions between the solutes and the membrane can play a role, such as Donnan exclusion based on electric charge. In water treatment, UF is used to replace filtration of suspended solids and for disinfection.
NF and RO membranes are composite and they require a larger hydraulic pressure to drive the separation, see figure above. NF membranes allow salts and other small molecules to pass through but retain larger molecules such as peptides, hormones, and sugars. NF is therefore deployed to remove hardness or pharmaceuticals from drinking water sources. In RO membranes, the pores are so small — from 0.3 to 0.5 nm in diameter — that they are within the range of thermal motion of the polymer chains that form the membrane. RO membranes allow water to go through but retain all dissolved species present in the feed, and they are thus used for desalination. The accepted mechanism of transport through these dense composite membranes is called solution-diffusion [6]. According to this model, solutes permeate the membrane by dissolving in the membrane material and diffusing down a concentration gradient. Separation is thus rate-based and it occurs because of the difference in solubilities and mobilities of compounds in the membrane. For more details about membrane transport theory and examples, we suggest looking at the available literature [1,6].
Membrane processes. Membrane processes are divided based on the membrane type, what it separates, the driving force used to create the chemical potential difference between the two sides of the membrane, and the transport mechanism [1,2]. Here, we will focus on separation driven by a hydraulic trans-membrane pressure. Microfiltration (MF) and ultrafiltration (UF) are similar in that the separation is mostly molecular sieving through the pores. These membranes are illustrated in the right figure. MF membranes are usually symmetric and can filter colloidal particles and bacteria from 0.1 to 10 μm in diameter. Therefore, they are used for clarification, sterilization, and to concentrate suspensions. In water treatment, MF is used to remove pathogens for disinfection and occasionally to remove turbidity and flocculants in wastewater treatment. UF membranes are generally asymmetric and they can be used to filter dissolved macromolecules, such as proteins, from solutions. While the primary separation mechanism in UF is size exclusion, other physicochemical interactions between the solutes and the membrane can play a role, such as Donnan exclusion based on electric charge. In water treatment, UF is used to replace filtration of suspended solids and for disinfection.
NF and RO membranes are composite and they require a larger hydraulic pressure to drive the separation, see figure above. NF membranes allow salts and other small molecules to pass through but retain larger molecules such as peptides, hormones, and sugars. NF is therefore deployed to remove hardness or pharmaceuticals from drinking water sources. In RO membranes, the pores are so small — from 0.3 to 0.5 nm in diameter — that they are within the range of thermal motion of the polymer chains that form the membrane. RO membranes allow water to go through but retain all dissolved species present in the feed, and they are thus used for desalination. The accepted mechanism of transport through these dense composite membranes is called solution-diffusion [6]. According to this model, solutes permeate the membrane by dissolving in the membrane material and diffusing down a concentration gradient. Separation is thus rate-based and it occurs because of the difference in solubilities and mobilities of compounds in the membrane. For more details about membrane transport theory and examples, we suggest looking at the available literature [1,6].
Some general guidelines for composite membrane selectivity can be summarized as follows: (i) multivalent ions are retained better than monovalent ions. Although the absolute values of the salt rejection vary over a wide range, the ranking for the different salts is the same for all membranes. In general, for cations rejection follows: Fe2+ > Ni2+ ~ Cu2+ > Mg2+ > Ca2+ > Na+ > K+, and for anions: PO43– > SO42– > HCO3– > Br– > Cl3– > NO3– ~ F–. (ii) Dissolved gases such as ammonia, carbon dioxide, sulfur dioxide, oxygen, chlorine, and hydrogen sulfide always permeate well. (iii) Rejection of weak acids and bases is pH dependent. When the acid or base is in the ionized form the rejection will be high, but in the non-ionized form rejection will be low. (iv) Rejection of neutral organic solutes generally increases with the molecular weight (or diameter) of the solute.
There are numerous technologies based on membrane separation. After a brief overview of fouling mechanisms and possible solutions to mitigate fouling, we will narrow our focus on processes for water and wastewater treatment that are being promisingly developed to improve existing treatment schemes or to increase water supply beyond what is available from the hydrological cycle. In particular, we will discuss wastewater recycling using membrane bioreactors, and seawater desalination with reverse osmosis. We will briefly illustrate the potential of these two applications, as well as the current challenges related to their full-scale implementation.
Membrane fouling and cleaning
The major obstacle for the application of membrane processes is the rapid decline of the permeate flux over time as a result of membrane fouling. Fouling of membranes is caused by mass transport of material to the membrane surface followed by adsorption and accumulation onto the surface and/or within membrane pores [7].
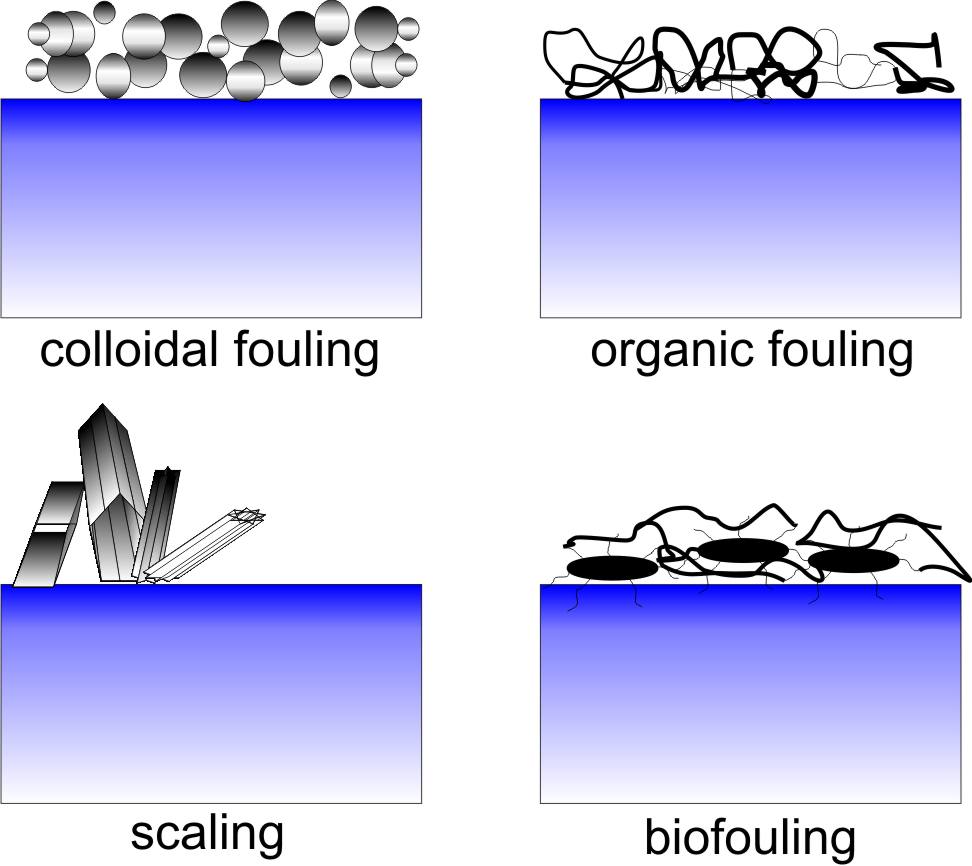 Types of foulant. Raw waters contain a wide distribution of foulants, see right figure. Particulate fouling is caused by inorganic or organic particles that can deposit on the membrane surface, block the pores, or hinder transport to the surface by the development of a cake layer. Aquatic colloids comprise corrosion products, silt and clay, precipitated crystals, colloidal silica and sulfur, and precipitated iron and aluminum compounds. Some high molecular weight organic substances, such as polysaccharide, proteins, and humic aggregates, are also characterized as colloidal foulants since many features of their behavior are common with those of inorganic particles. The structure and mass of the deposited particulate cake layer are affected by the particle-particle forces and by hydrodynamic conditions [8]. For example, under chemical conditions in which particles repel each other and colloidal stability is maintained, the cake layer is generally more porous, inducing lower permeate flux decline. Such a cake layer will also be easily removed from the membrane surface during cleaning.
Types of foulant. Raw waters contain a wide distribution of foulants, see right figure. Particulate fouling is caused by inorganic or organic particles that can deposit on the membrane surface, block the pores, or hinder transport to the surface by the development of a cake layer. Aquatic colloids comprise corrosion products, silt and clay, precipitated crystals, colloidal silica and sulfur, and precipitated iron and aluminum compounds. Some high molecular weight organic substances, such as polysaccharide, proteins, and humic aggregates, are also characterized as colloidal foulants since many features of their behavior are common with those of inorganic particles. The structure and mass of the deposited particulate cake layer are affected by the particle-particle forces and by hydrodynamic conditions [8]. For example, under chemical conditions in which particles repel each other and colloidal stability is maintained, the cake layer is generally more porous, inducing lower permeate flux decline. Such a cake layer will also be easily removed from the membrane surface during cleaning.
Organic fouling is the result of the adsorption of dissolved components [9]. Dissolved organic matter is ubiquitous in surface water, sewage, and secondary effluent of wastewater treatment. In drinking water treatment by MF and UF, natural organic matter (NOM) has been identified as a major foulant of polymeric membranes. NOM comprises a range of compounds, from small hydrophobic acids, proteins and amino-acids to larger humic and fulvic acids. There is evidence that the larger, less charged, and the more hydrophobic fraction of NOM mostly contributes to irreversible organic fouling. This mechanism is consistent with the general phenomenon of adsorption of polyelectrolytes and polymers on solid substrates, which is faster and more pronounced in case of non-repulsive polymer-surface electrostatic interactions and due to hydrophobic forces. Adlayers of polymers are usually irreversibly attached, such that chemical cleaning is necessary to induce desorption.
Scaling is another type of fouling related to dissolved ions that tend to precipitate onto the membrane surface due to pH change or due to oxidation. Deposition is followed by crystallization and crystal growth. Compounds commonly present in feed water and with a low solubility are calcium carbonate (CaCO3), barium sulfate (BaSO4), silica (SiO2), and calcium sulfate (CaSO4). Antiscalant addition to the feed solution leads to a decline in the percent of area covered by scale.
Finally, biofouling is caused by microbiological foulants, such as algae and bacteria, which can adhere to the membranes and produce a biofilm [10]. Biofouling involves accumulation of these biological organisms, their growth and metabolism on the membranes. Components in the biofilm are the cell biomass and the various extracellular polymeric substances, which behave in the same way as organic foulants. In all biofilms, the fraction of organic macromolecules is usually the largest, accounting for 50-80% of the total organic matter and proteins. Biofouling is arguably the major challenge when using RO for the reclamation of municipal effluents or for seawater desalination. Obviously, the extent and the type of fouling depend strongly on the quality of the feed water and on operating conditions. Especially in the case of complex water sources, different types of foulants will necessarily contribute simultaneously to the formation of a mixed fouling layer.
Antifouling membranes. Efforts to mitigate fouling include pretreatment processes, the design of new membrane modules, and the development of antifouling membranes. The separation process by membrane is essentially a surface phenomenon. Therefore, it is a natural consequence to modify membrane surface for reducing fouling. The membrane surface can be customized to tailor the following properties [11,12]:
Surface hydrophilicity: it is generally accepted that an increase in hydrophilicity offers better fouling resistance because many foulants, such as proteins, are hydrophobic in nature [13].
Surface charge: the repulsive forces working between the charged surface and the molecules of the same charge in the feed solution prevent solute or particle deposition on the membrane surface, thus reducing fouling. In reality, when the ionic strength of the feed water is high, electrostatic interactions are minimized, rendering the surface charge ineffective in terms of electrostatic repulsion. On the contrary, a detrimental phenomenon called bridging can occur if both the membrane surface and the foulant molecule contain negatively-charged carboxyl groups [14]. Bridging is caused by divalent calcium ions in solution cross-linking the carboxyls of the membrane surface with the carboxyl groups of the fouling molecules, thus enhancing the attachment of these molecules to the membrane surface. Polyamide membranes contain inherent carboxyls at their surface, making this mechanism common during RO and NF operation.
Surface roughness: a smoother surface is commonly expected to experience less fouling, presumably because foulant particles are more likely to be entrained by rougher topologies than by smoother membrane surfaces.
The effect of membrane surface modification on fouling is actually a debated topic. Some researchers have experienced that membrane fouling can be reduced by modifying the membrane surface only when the solution is dilute or only in the initial stage of the separation experiment. Once the deposition of foulants has taken place, the surface properties can no longer play a role in further deposition of foulants, which is then governed by foulant-foulant interactions. One route for fouling prevention is to develop fouling release membranes that do not resist the adhesion of foulants, but have an active layer with a low surface energy so that adhered foulants can readily be washed away by hydrodynamic mixing in the membrane module. However, a major challenge is to implement these chemistries such that the water flux and salt rejection of the resulting membranes are not compromised [11].
Membrane cleaning. Once a fouling layer has developed, cleaning is necessary. Membrane fouling can be classified as reversible fouling and irreversible fouling, of which the distinction is entirely dependent on the context in which membranes are operated and cleaned. An easily removable outer fouling layer is usually categorized as reversible. The performance of a membrane with reversible fouling can be restored through appropriate physical washing protocol such as backwashing or surface washing, while irreversible fouling can only be removed by chemical cleaning, and sometimes cannot be removed at all. This means that the membranes must go through extensive cleaning or be replaced.
Physical cleaning can be performed relatively often and involves a simple change in the physical conditions of the system, for example increasing the hydrodynamics at the membrane-solution interface by changing the flow conditions or by inserting air bubbles. Sometimes, even simple changes in chemical conditions, such as temporary substitution of the feed solution with a foulant-free solution — often the permeate itself — is regarded as physical cleaning. However, irreversible fouling is inevitable. The long-term solution is to remove the foulant deposited on membrane surfaces via chemical cleaning. There are five categories of cleaning agents — alkaline solutions, acids, metal chelating agents, surfactants, and enzymes [15]. Commercial cleaning products are often mixtures of these compounds. Alkaline solutions clean organic-fouled membranes by hydrolysis and solubilization. Alkaline solutions increase the solution pH, and therefore increase the negative charge and solubility of the organic foulant. For example, when carboxylic functional groups of the organic foulant are completely deprotonated under alkaline conditions, solubility increases by few orders of magnitude. In the presence of divalent cations, such as Ca2+, carboxyl-rich foulants forms complexes with calcium ions, resulting in a highly compacted gel network of fouling layer. Metal chelating agents, such as ethylenediaminetetraacetic acid (EDTA), remove divalent cations from the complexed organic molecules and improve the cleaning of the fouled membrane. Finally, surfactants can form micelles around macromolecules, solubilize them and help to remove the foulant from the membrane surface. It has been shown that cleaning efficiencies with different cleaning agents are consistent with related measurements of foulant-foulant intermolecular forces in the presence of the agents, using the atomic force microscopy [16]. Virtually, the optimal cleaning agent mixture and concentration could be derived from measurements of foulant-foulant adhesion force, even before membranes are put in operation. For favorably reactive cleaning agents, cleaning efficiency can be further improved by enhancing the mass transfer of the reaction products from the fouling layer to the bulk solution. Nevertheless, repeated chemical cleaning will eventually result in wear and tear and eventual loss of membrane properties.
Wastewater recycling using membrane bioreactors
By recycling wastewater, the circulation of water through the natural water cycle can be short-circuited, such that production of water for human needs has a smaller impact on environmental resources. Reuse encompasses any type of beneficial use of recycled water, from restricted agricultural irrigation all the way to unrestricted residential use for potable water [17]. Wastewater treatment is based on the removal of solids in suspension, soluble organic compounds, and nutrients, such as phosphorus and nitrogen. A common way to degrade and remove most of the contaminants from wastewater is using the activated sludge process. This technology consists in biodegradation of the pretreated influent by microorganisms followed by a clarification in a settling tank to ensure the separation of the treated water from the biomass. Although this technique may appear robust and safe, the treated water quality is dependent on the settling properties of the biological suspension and the treatment efficiency is usually limited by the difficulties in separating suspended solids [18]. Furthermore, this process requires large aeration and sedimentation tanks, and it generates large quantities of sludge. Membrane technology can play an important role to overcome some of the problems associated with traditional processes, such as activated sludge.
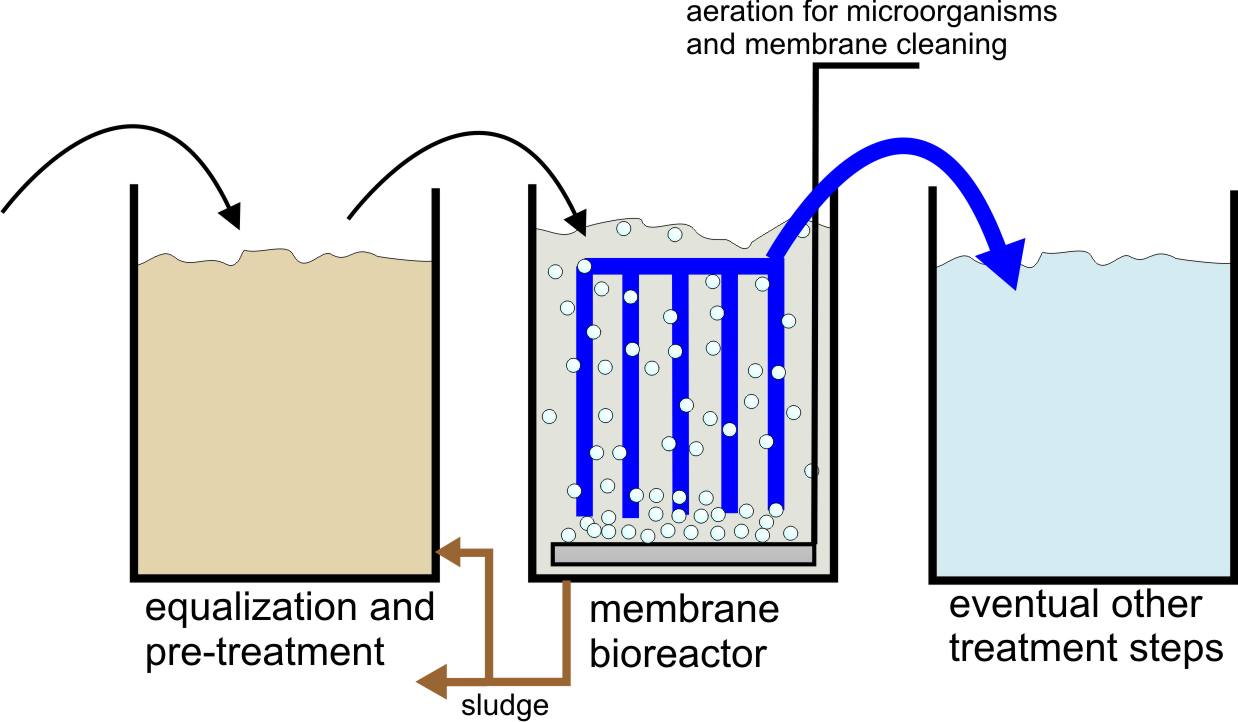
The membrane bioreactor (MBR). Membrane bioreactors (MBR) are an improvement of the activated sludge process, replacing the two stages of the conventional process — biological treatment and clarification — with a single, integrated biotreatment and clarification step, see figure on the next page [18,19]. In this process, membranes are directly immersed within the biological reactor. This tank is completely mixed and the liquor is filtered through the membrane, thus producing a high quality effluent. The membrane surface also acts as a substrate for the development of a biofilm, which further helps the rejection of viruses and other compounds, which otherwise would freely permeate across the porous membranes. Aeration within the reactor provides oxygen to allow aerobic biotransformation and helps minimizing membrane fouling through the resulting shear. By using MF or UF, MBRs allow the complete physical retention of bacterial flocs and virtually all suspended solids within the bioreactor. There is evidence of significant retention of other associated pollutants, such as heavy metals and micropollutants, although not always below the required concentrations. Removal of these contaminants is allowed by the long sludge retention time. Since degradation in MBR is largely dominated by the biological component, bioaugmentation helps achieving higher removal, which is related to the biodegradability of the individual compound. Due to the absence of secondary clarifier and the possibility to use a high sludge concentration, the overall size of the treatment plant can be reduced significantly if MBR is used instead of activated sludge.
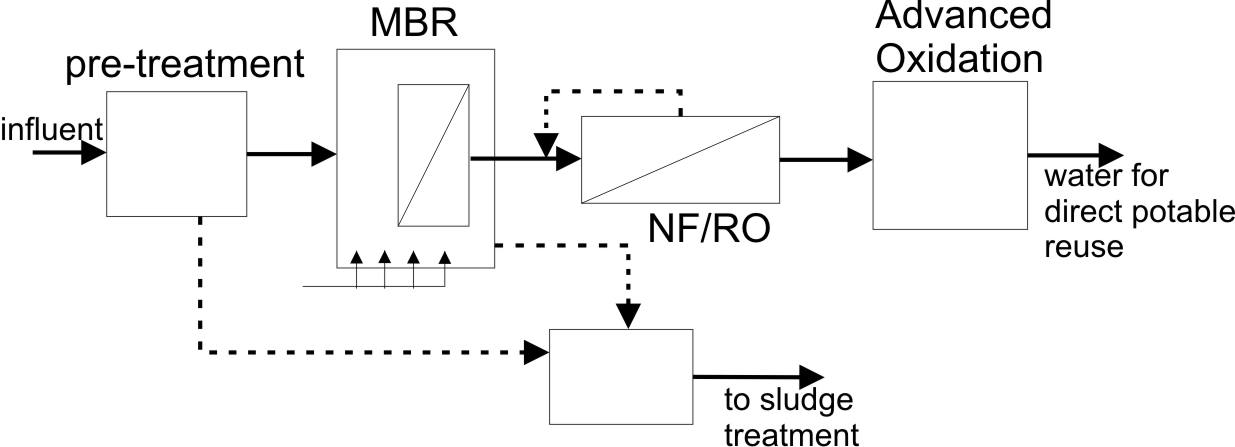
Membrane-based wastewater recycling. The advantages of MBR in terms of flexibility and quality of the treated water suggest that this process can be used to produce water to be directly reused for irrigation, cleaning or cooling on industrial site, and domestic purposes [18]. However, MBR can also be thought as an excellent pretreatment step before further treatment for the production of water for direct potable reuse [17,18]. For unrestricted reuse, MBR is always followed by other separation steps, such as activated carbon, advanced oxidation, and/or a more selective membrane step. Given the complementary treatment capacity of MBR and NF/RO membrane filtration, there is potential for the coupling of these two treatment processes to achieve an overall higher performance, see figure on the next page [20]. Authors reported MBR-RO studies, demonstrating a consistently high treated water quality, using highly contaminated feed with inorganic, organic, and microbiological contaminants [21]. In addition, it has been reported that the deployment of MBR prior to RO filtration could reduce the fouling of the RO membrane [20].
The combination of MBR and NF/RO treatments for wastewater reuse remains very uncommon in practice. The limiting factor remains the membrane fouling that reduces the membrane permeability during the MBR operation. As the feed water source contains a high level of a wide range of different contaminants, membrane fouling is a complex and site specific phenomenon [18,22]. Three main families of compounds — biological, particulate, and dissolved organic compounds — take part in fouling of MF or UF membranes within MBRs [22]. Initial adsorption and pore blocking by colloids, organic matter, and extracellular polymeric substances serve as conditioning fouling, which is usually irreversible. Subsequently, biofilm growth and cake formation further decrease the system productivity, but can be considered as reversible fouling. Deposition of solids and high-molecular weight compounds can be usually controlled during the operation by means of high shear stress at the membrane surface, relaxation periods, or back flushing [18]. The fabrication of antifouling membranes would virtually allow the development of a single-stage MBR with an immersed tight nanofiltration membrane, which would replace the two stages of MBR and NF/RO processes [20]. Recently, osmotic MBR technology based on forward osmosis has been suggested to mitigate fouling and to enhance the overall process performance [23,24].
Case study. An interesting case study about the implementation of MBR technology for the reuse of wastewater is that of Japan. Initially, the Japanese government promoted the reclamation of wastewater in the mega cities of Fukuoka and Tokyo. City legislation required large buildings and commercial facilities to adopt water saving measures including rainwater harvesting and in-building greywater treatment and reuse systems. This initiative was followed by the issue of strict water quality standards for reclaimed wastewater, which required the adoption of advance technologies for reclamation. The Aqua Renaissance program '90 led to development of MBR systems by companies such as Kubota and Mitsubishi, resulting in the later demonstration and adoption of MBR processes both in remodeled existing facilities and in new satellite sewage treatment, within the project A-JUMP. Here, MBR processes have shown significant advantages over alternative biological treatment processes, particularly in terms of pathogen removal and process robustness. Nowadays, a high percentage of the worldwide MBR systems are installed in Japan for in-building greywater treatment, with the aim of restricted residential use.
While wastewater reuse represents one of possible solutions to the growing pressure on water resources, its implementation is hindered by technical, economical, and legislation issues, as well as public awareness and, most importantly, acceptance. Some authors have underlined that we are technically capable of treating wastewater to any quality we desire simply by "filtering it through money" [17]. If money is available, technology usually allows the production of safe water with negligible levels of any contaminants. However, the level of treatment will be compromise between the nature and concentration of contaminants, the specific beneficial use of the treated water, and the related water quality standards and treatment costs to achieve them. Furthermore, reuse of wastewater usually requires major capital investment, not only related to the treatment facility, but associated to the installation of a dual piping system, to careful control of effluent quality, and to additional precautions to minimize health and environmental risks [17]. Therefore, beneficial recycling of wastewater relies on a paradigm shift in the way we think about waste and commodity, and it is a political issue as much as it is a scientific challenge.
Seawater desalination using reverse osmosis
Reverse osmosis desalination scheme. Membrane desalination by reverse osmosis is the fastest growing desalination technology. Seawater has a salt concentration of 32-40 g/L, depending on the region of the world. The osmotic pressure of seawater is about 350 psi (24 bar), and the osmotic pressure of the rejected brine can be as much as 600 psi (41 bar), so osmotic pressure affects the net operating pressure in a plant markedly. Currently, all new seawater desalination plants are based on interfacial composite membranes with the dense layer made of polyamide of the fully aromatic type. Typical thin-film composite polyamide membranes, tested with 3.5% sodium chloride solutions, have a salt rejection of 99.5% and a water flux of 0.5 L m-2h–1 at 800 psi [11]. The key steps of a desalination system are shown in the figure below.
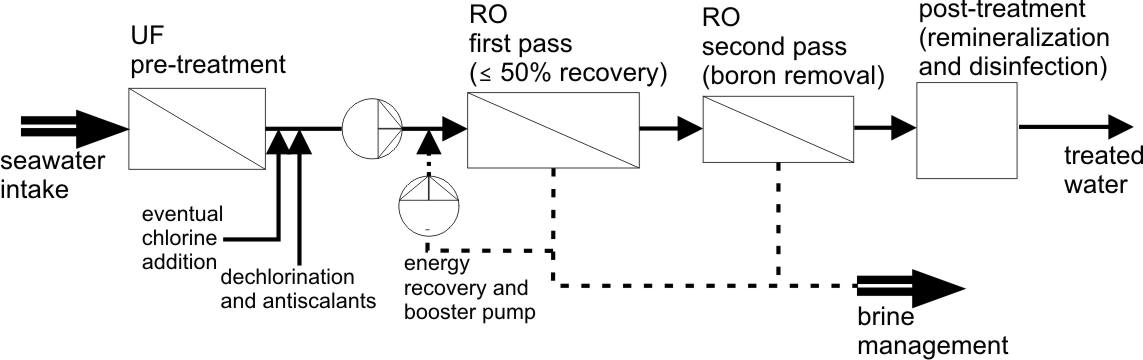
These steps are (i) seawater intake, including the structures and strategies used to extract seawater and convey it to the process system, (ii) pretreatment, defined as the removal of suspended solids and control of biological growth, often performed using UF: this step may entail addition of chlorine-based disinfectants and subsequent dechlorination before the desalination step, as well as addition of antiscalants; (iii) desalination, the process that removes dissolved ions in RO: removal of boron below the required concentration may require a second RO pass; (iv) post-treatment, including the addition of chemicals to the product water to prevent corrosion of downstream infrastructure piping and to improve taste; (v) brine discharge and management, the handling and disposal or reuse of waste residuals from the desalination system. Utilization of the residual pressure of the brine leaving the RO membrane module is a necessary step to recover otherwise wasted discharged energy.
 Energy input of seawater desalination by RO.
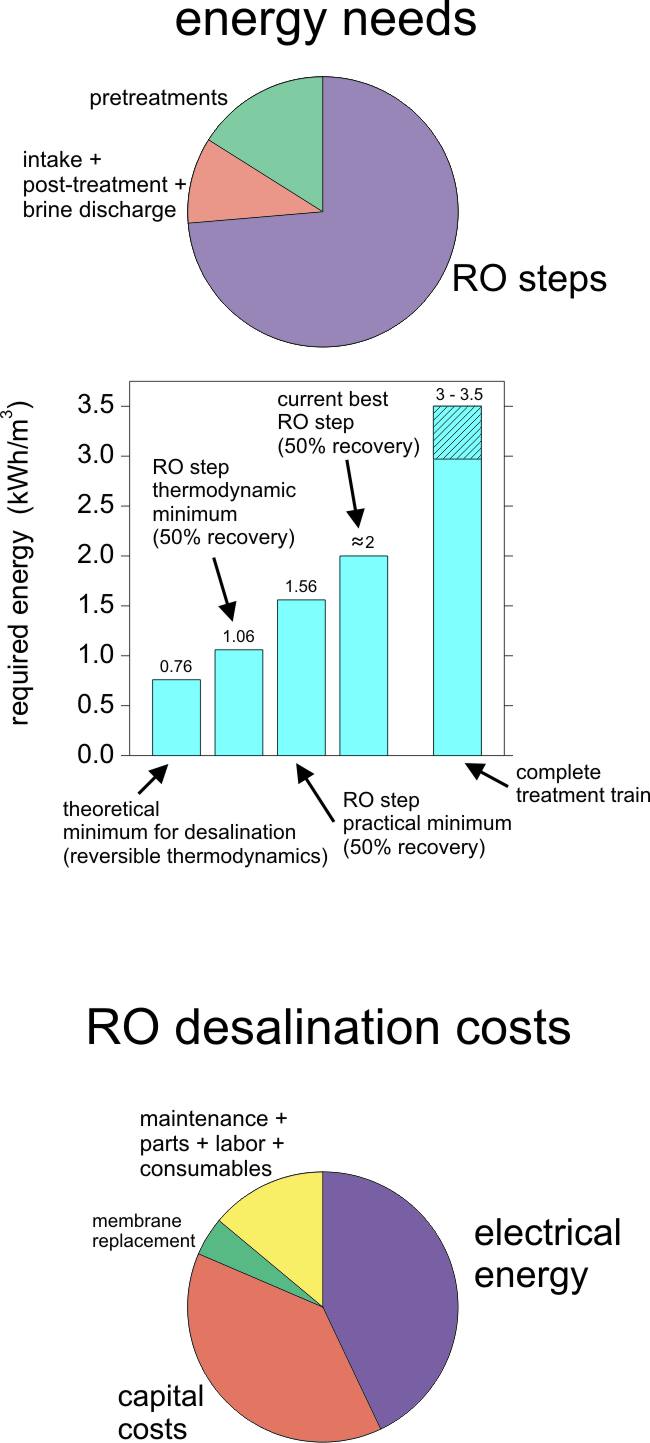
The minimum amount of energy required to separate pure water from seawater is a benchmark for comparison of the efficiency of current desalination schemes and can guide efforts to reduce energy demand [11]. This theoretical minimum energy, which is independent of the desalination method, is realized when the separation occurs as a reversible thermodynamic process. When two solutions of different composition are mixed (or separated), the Gibbs free energy of mixing is released (or needed). Separating an infinitely small amount of pure water from seawater requires approximately 0.76 kWh/m3 of work; see right figure. This work is equivalent to the minimum energy to desalinate seawater at 0% recovery. If the salinity of seawater or if the desired water recovery increases, so does the minimum energy required for desalination. The change in Gibbs free energy represents a lower bound on the energy that is needed to desalinate, regardless of the pathway. However, the second law of thermodynamics dictates that the actual energy needed is always more than the theoretical one. In the theoretical reversible thermodynamic RO model, an infinitesimal water flux is maintained throughout the process. This is achieved by virtually applying a hydraulic pressure negligibly larger than the osmotic pressure difference on the feed solution such that an infinitesimally small volume of pure water permeates across the membrane. In this reversible thermodynamic RO process, no entropy is generated. For a usual 50% recovery, the ideal energy value required to achieve desalination equals 1.06 kWh/m3. Unlike in the reversible RO process, a net constant driving pressure equal to the final osmotic pressure of the concentrated feed is required to maintain a nonzero permeate flux. When the applied pressure is equal to the osmotic pressure of the concentrate, the system is said to be operating at the thermodynamic limit. Therefore, regardless of how permeable a membrane is, the energy consumption is set by the need to bring the feed volume to a pressure equal to the osmotic pressure of the concentrate [11]. The RO desalination step at 50% recovery has a practical minimum energy of 1.56 kWh/m3. Thus, 0.5 kWh/m3 is expended because the system has a finite size and is not operating as a reversible thermodynamic process. In reality, an extra amount of energy is needed to overcome concentration polarization. This ideal energy consumption of 1.56 kWh/m3 is not far from reported energy consumptions of 2–2.5 kWh/m3 of current well-designed RO systems, see figure on the previous page.
Energy input of seawater desalination by RO.
The minimum amount of energy required to separate pure water from seawater is a benchmark for comparison of the efficiency of current desalination schemes and can guide efforts to reduce energy demand [11]. This theoretical minimum energy, which is independent of the desalination method, is realized when the separation occurs as a reversible thermodynamic process. When two solutions of different composition are mixed (or separated), the Gibbs free energy of mixing is released (or needed). Separating an infinitely small amount of pure water from seawater requires approximately 0.76 kWh/m3 of work; see right figure. This work is equivalent to the minimum energy to desalinate seawater at 0% recovery. If the salinity of seawater or if the desired water recovery increases, so does the minimum energy required for desalination. The change in Gibbs free energy represents a lower bound on the energy that is needed to desalinate, regardless of the pathway. However, the second law of thermodynamics dictates that the actual energy needed is always more than the theoretical one. In the theoretical reversible thermodynamic RO model, an infinitesimal water flux is maintained throughout the process. This is achieved by virtually applying a hydraulic pressure negligibly larger than the osmotic pressure difference on the feed solution such that an infinitesimally small volume of pure water permeates across the membrane. In this reversible thermodynamic RO process, no entropy is generated. For a usual 50% recovery, the ideal energy value required to achieve desalination equals 1.06 kWh/m3. Unlike in the reversible RO process, a net constant driving pressure equal to the final osmotic pressure of the concentrated feed is required to maintain a nonzero permeate flux. When the applied pressure is equal to the osmotic pressure of the concentrate, the system is said to be operating at the thermodynamic limit. Therefore, regardless of how permeable a membrane is, the energy consumption is set by the need to bring the feed volume to a pressure equal to the osmotic pressure of the concentrate [11]. The RO desalination step at 50% recovery has a practical minimum energy of 1.56 kWh/m3. Thus, 0.5 kWh/m3 is expended because the system has a finite size and is not operating as a reversible thermodynamic process. In reality, an extra amount of energy is needed to overcome concentration polarization. This ideal energy consumption of 1.56 kWh/m3 is not far from reported energy consumptions of 2–2.5 kWh/m3 of current well-designed RO systems, see figure on the previous page.
Costs of RO seawater desalination. The surface properties of thin-film composite polyamide membranes make them prone to fouling, which diminishes process performance. Biofouling is the fouling mechanisms that mostly impair the performance of the RO desalination step. Biofilm formation could potentially be prevented if chlorine or other oxidants were added to the feed. However, polyamide films undergo a permanent loss in performance resulting from exposure to even ppb levels of these disinfectants. Therefore, good pretreatment of the raw water before it is fed into the RO stage is essential. While the energy demand for seawater desalination by state-of-the-art reverse osmosis is not far from the theoretical minimum energy for desalination, the overall energy consumption of overall RO plants is roughly four times higher than this theoretical minimum due to the need for extensive pretreatment, which account for almost 1 kWh/m3. The development of fouling-resistant membranes would improve the energy efficiency of desalination by RO. However, no membrane yet exists that possesses both high separation performances and antifouling properties [11]. Energy is the largest single variable cost for desalination as shown in the figure on the previous page. Ashkelon desalination plant, the largest seawater RO plant in the world, was built at a cost of more than USD 200.000.000, and produces drinking water at a price of approximately USD 0.6 USD/m3.
Seawater desalination, like any other major industrial process, has environmental impacts that must be understood and mitigated. These impacts include effects associated with the construction of the plant but, especially, the effects of withdrawing large volumes of seawater from the ocean and discharging large volumes of highly concentrated brine. There is presently a considerable amount of uncertainty about the environmental impacts of desalination. Each desalination facility must be individually evaluated in the context of location, plant design, and local environmental conditions.
List of Acronyms
| MBR | Membrane bioreactor | NOM | Natural organic matter |
| MF | Microfiltration | RO | Reverse osmosis |
| NF | Nanofiltration | UF | Ultrafiltration |
Alberto Tiraferri
Email. alberto.tiraferri@unige.ch
Direct link www.colloid.ch/membranes
First posted, September 21, 2014, last revision, September 21, 2014
This work is licensed under a Creative Commons Attribution 4.0 International License.
References
[1] Baker R. (2004) Membrane technology and applications, 2nd ed., Wiley.
[2] Mulder J. (1996) Basic principles of membrane technology. 2nd ed., Springer.
[3] Tiraferri A., Yip N. Y., Phillip W. A., Schiffman J. D., Elimelech M. (2011) Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure, J. Membrane Sci. 367, 340-352, 10.1016/j.memsci.2010.11.014.
[4] Wijmans J. G., Baaij J. P. B., Smolders C. A. (1983) The mechanism of formation of microporous or skinned membranes produced by immersion precipitation, J. Membrane Sci. 14, 263-274, 10.1016/0376-7388(83)80005-2.
[5] Petersen R. J. (1993) Composite Reverse-Osmosis and Nanofiltration Membranes, J. Membrane Sci. 83, 81-150, 10.1016/0376-7388(93)80014-O.
[6] Wijmans J. G., Baker R. W. (1995) The solution-diffusion model - a review, J. Membrane Sci. 107, 1-21, 10.1016/0376-7388(95)00102-I.
[7] Goosen M. F. A., Sablani S. S., Ai-Hinai H., Ai-Obeidani S., Al-Belushi R., Jackson D. (2004) Fouling of reverse osmosis and ultrafiltration membranes: A critical review, Sep. Sci. Technol. 39, 2261-2297, 10.1081/Ss-120039343.
[8] Song L. F., Elimelech M. (1995) Theory of concentration polarization in cross-flow filtration, J. Chem. Soc. Faraday Trans. 91, 3389-3398, 10.1039/Ft9959103389.
[9] Hong S. K., Elimelech M. (1997) Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes, J. Membrane Sci. 132, 159-181, 10.1016/S0376-7388(97)00060-4.
[10] Watnick P., Kolter R. (2000) Biofilm, city of microbes, J. Bacteriol. 182, 2675-2679, 10.1128/Jb.182.10.2675-2679.2000.
[11] Elimelech M., Phillip W. A. (2011) The future of seawater desalination: Energy, technology, and the environment, Science 333, 712-717, 10.1126/science.1200488.
[12] Rana D., Matsuura T. (2010) Surface modifications for antifouling membranes, Chem. Rev. 110, 2448-2471, 10.1021/Cr800208y.
[13] Tiraferri A., Kang Y., Giannelis E. P., Elimelech M. (2012) Superhydrophilic thin-film composite forward osmosis membranes for organic fouling control: Fouling behavior and antifouling mechanisms, Environ. Sci. Technol. 46, 11135-11144, 10.1021/es3028617.
[14] Mo Y. H., Tiraferri A., Yip N. Y., Adout A., Huang X., Elimelech M. (2012) Improved antifouling properties of polyamide nanofiltration membranes by reducing the density of surface carboxyl groups, Environ. Sci. Technol. 46, 13253-13261, 10.1021/es303673p.
[15] Ang W. S., Lee S. Y., Elimelech, M. (2016) Chemical and physical aspects of cleaning of organic-fouled reverse osmosis membranes, J. Membrane Sci. 272, 198-210, 10.1016/j.memsci.2005.07.035.
[16] Ang W. S., Tiraferri A., Chen K. L., Elimelech, M. (2011) Fouling and cleaning of RO membranes fouled by mixtures of organic foulants simulating wastewater effluent. J. Membrane Sci. 376, 196-206, 10.1016/j.memsci.2011.04.020.
[17] Wintgens T., Melin T., Schafer A., Khan S., Muston M., Bixio D., Thoeye C. (2005) The role of membrane processes in municipal wastewater reclamation and reuse, Desalination 178, 1-11, 10.1016/j.desal.2004.12.014.
[18] Wisniewski C. (2007) Membrane bioreactor for water reuse, Desalination 203, 15-19, 10.1016/j.desal.2006.05.002.
[19] Judd S. (2006) The MBR book, Elsevier.
[20] Shannon M. A., Bohn P. W., Elimelech M., Georgiadis J. G., Marinas B. J., Mayes A. M. (2008) Science and technology for water purification in the coming decades, Nature 452, 301-310, 10.1038/Nature06599.
[21] Alturki A. A., Tadkaew N., McDonald J. A., Khan S. J., Price W. E., Nghiem L. D. (2010) Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membrane Sci. 365, 206-215, 10.1016/j.memsci.2010.09.008.
[22] Le-Clech P., Chen V., Fane T. A. G. (2006) Fouling in membrane bioreactors used in wastewater treatment. J. Membrane Sci. 284, 17-53, 10.1016/j.memsci.2006.08.019.
[23] Achilli A., Cath T. Y., Marchand E. A., Childress A. E. (2009) The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 239, 10-21, 10.1016/j.desal.2008.02.022.
[24] Yap W. J., Zhang J. S., Lay W. C. L., Cao B., Fane A. G., Liu Y. (2012) State of the art of osmotic membrane bioreactors for water reclamation. Bioresource Technol. 122, 217-222, 10.1016/j.biortech.2012.03.060.
